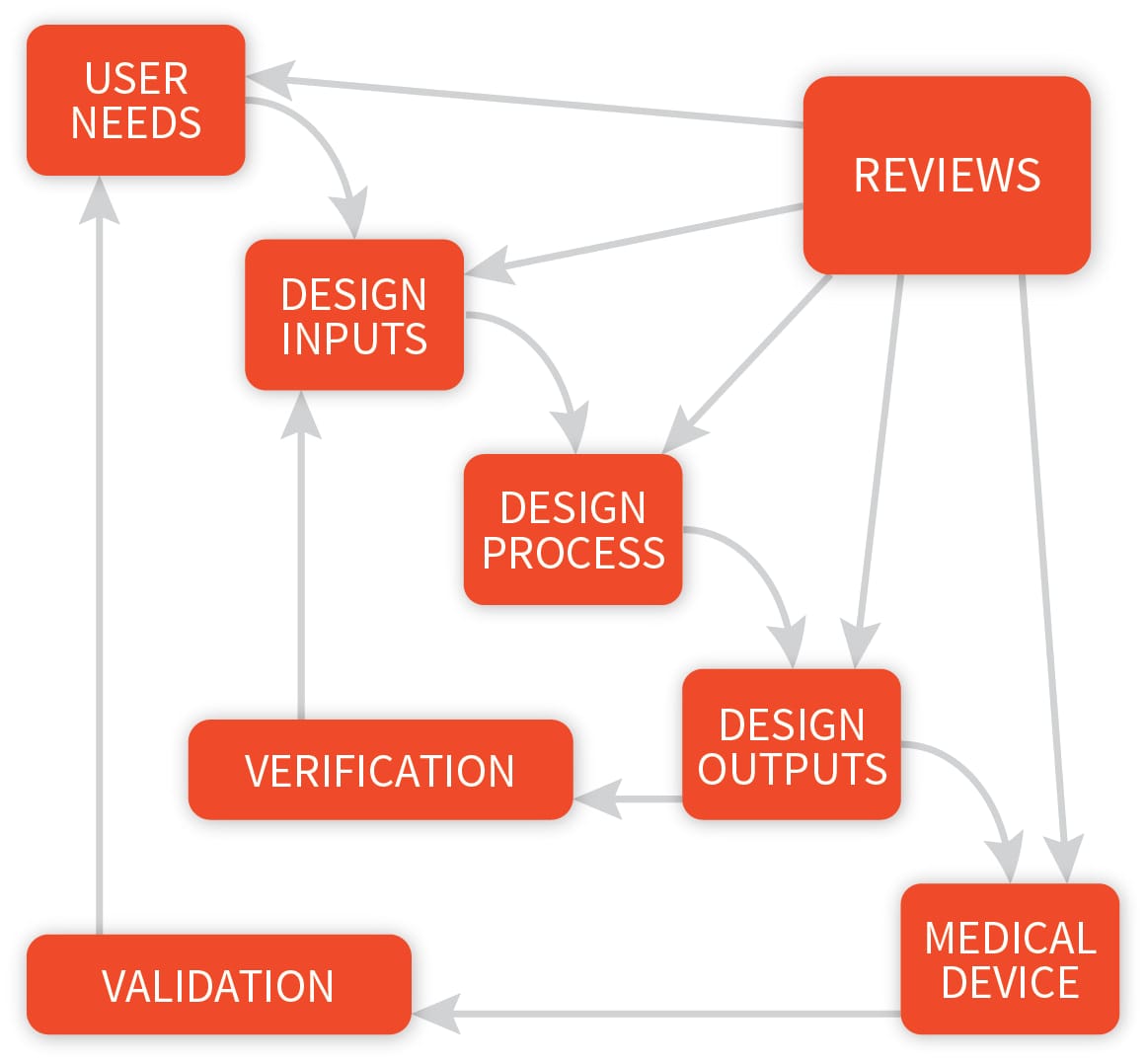
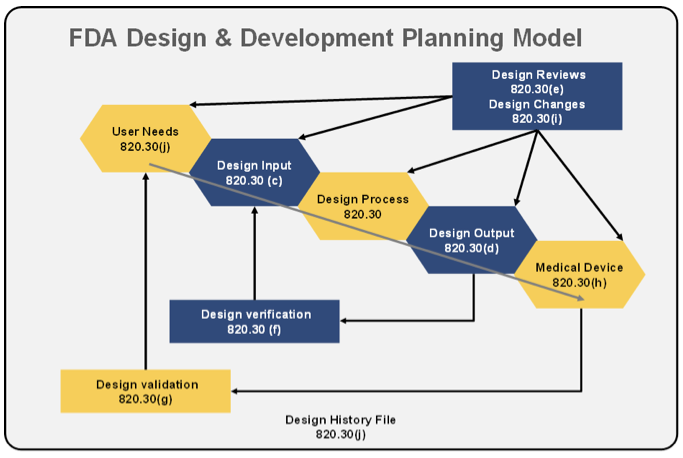
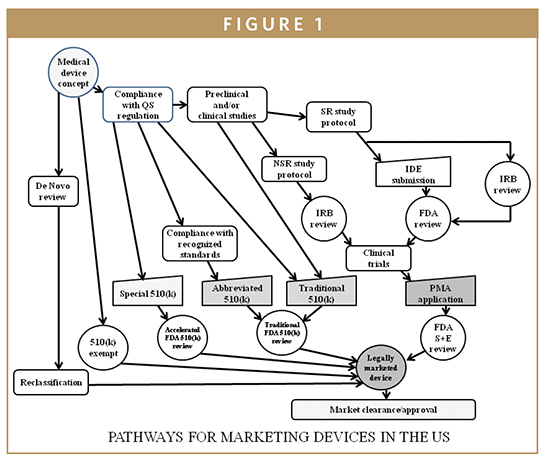
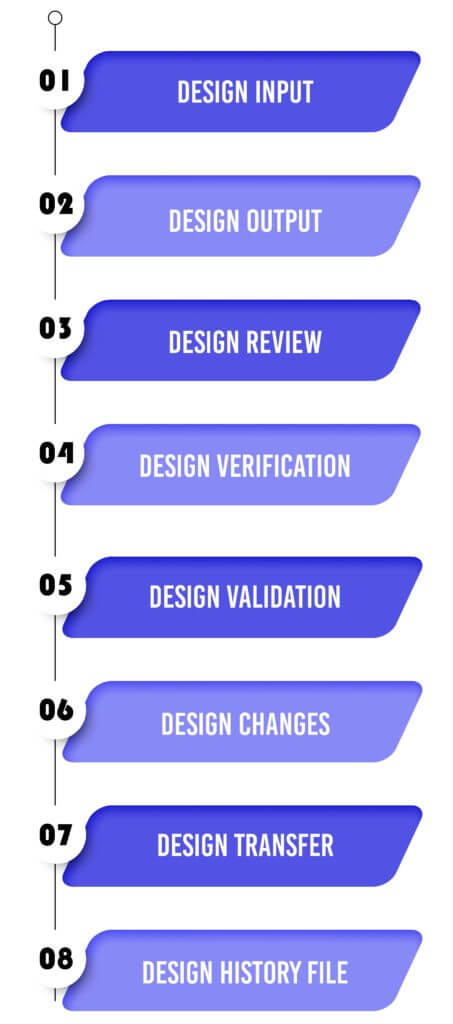
The steps in the FDA-mandated Design Control process (center section... | Download Scientific Diagram

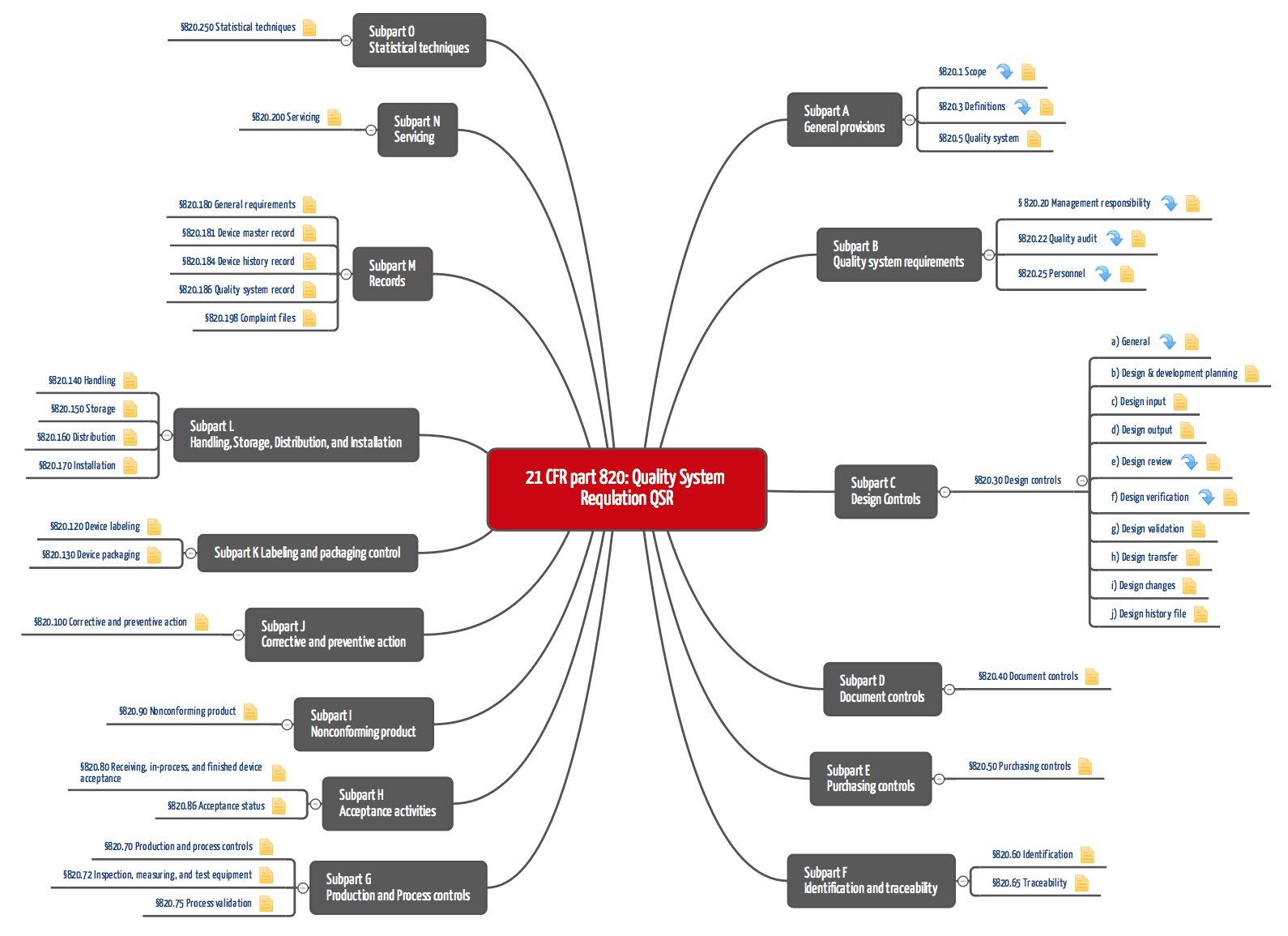
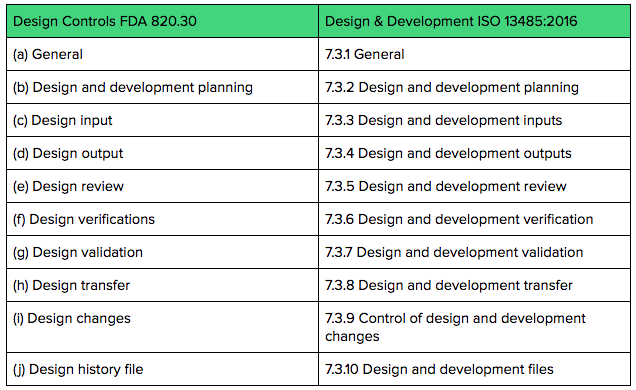
21 CFR Part 820 Subpart C – Design Controls - LearnGxP: Accredited Online Life Science Training Courses
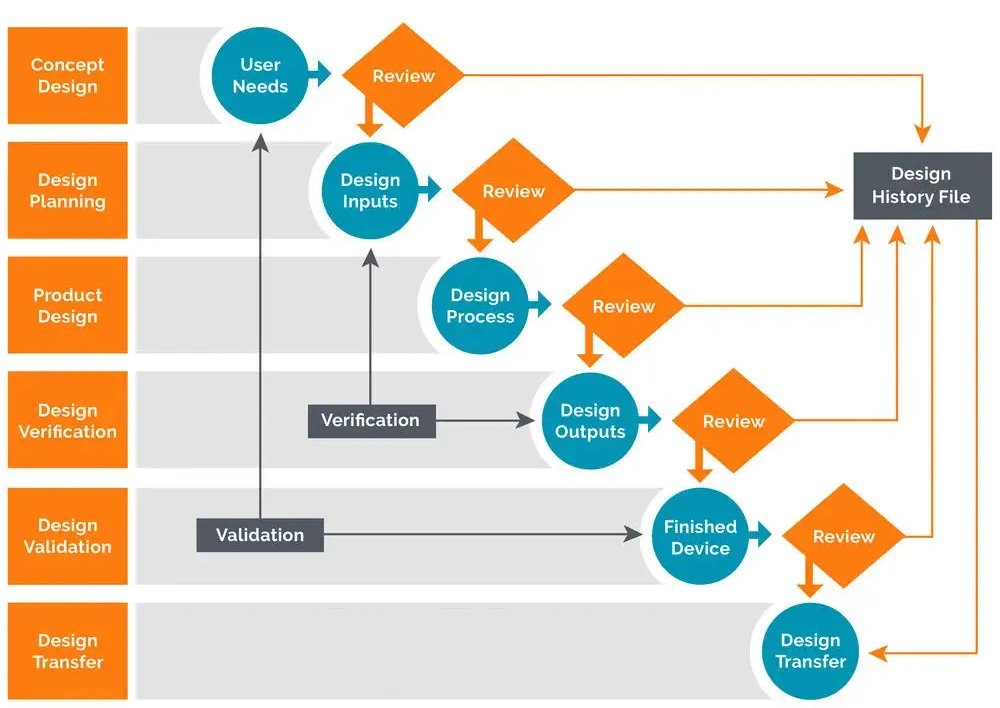
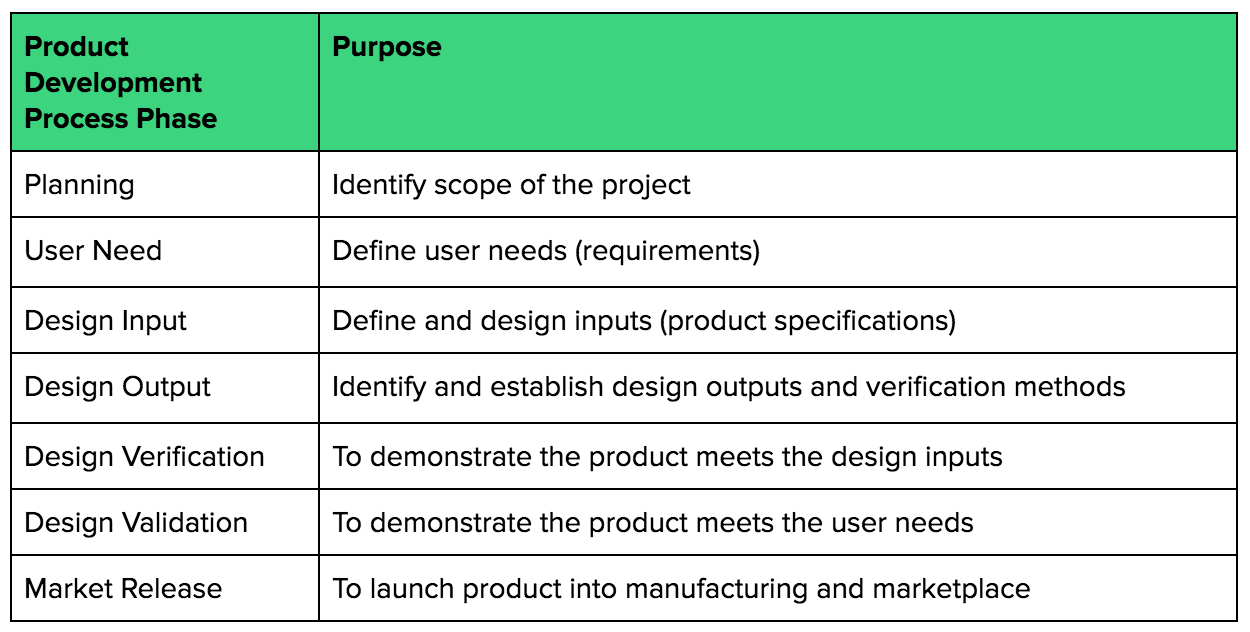
What is Design History File? Why it is Important for Medical Device Development | Medical Device - Johari Digital Healthcare Ltd.

21 CFR Part 820 - Quality System Regulation | 21 CFR 820.30 Medical Device Design Control Guidelines - YouTube

Agile Development in Regulated Environments Example: Medical Devices – Waterfall Lifecyle Model | Scaling Software Agility
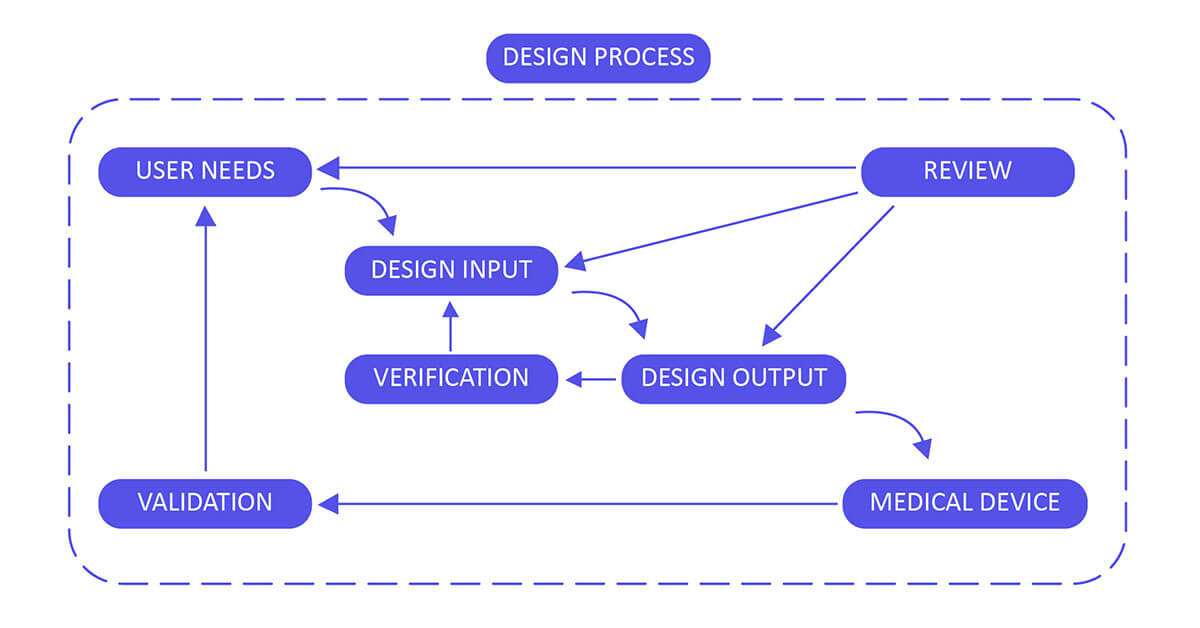
Application of design controls to design process. Design review should... | Download Scientific Diagram

Agile Development in Regulated Environments Example: Medical Devices – Waterfall Lifecyle Model | Scaling Software Agility

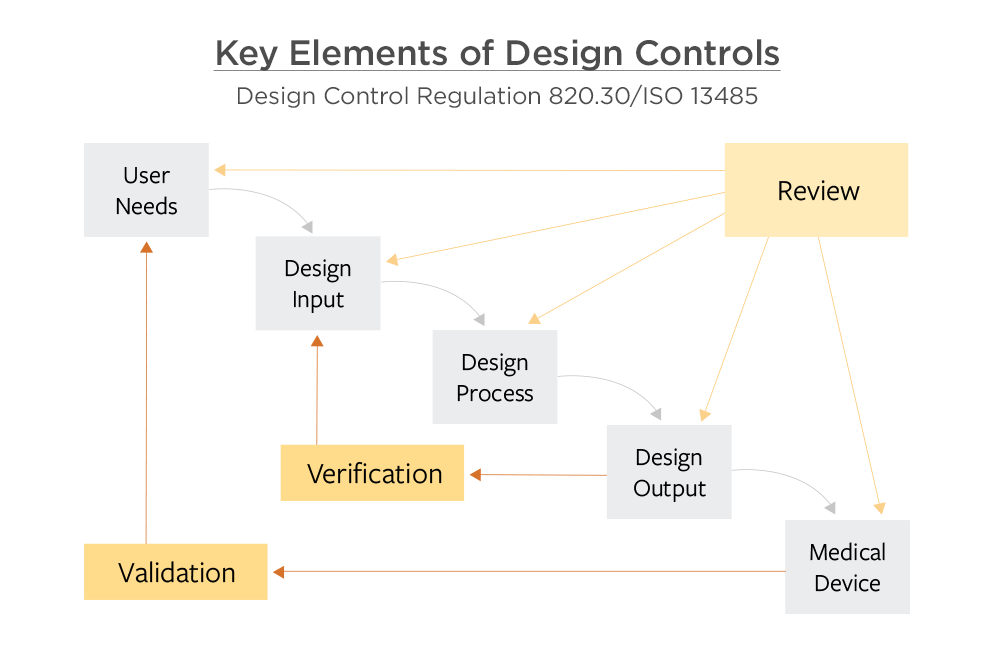
US FDA 21 CFR 820.30 (Documentation and Process for Design Controls For Medical Devices) | Operon Strategist | Isometric, Medical, Online registration

Design Controls: Building Objective Evidence and Process Architecture to Meet FDA and ISO Compliance - OMTEC 2018 | PPT


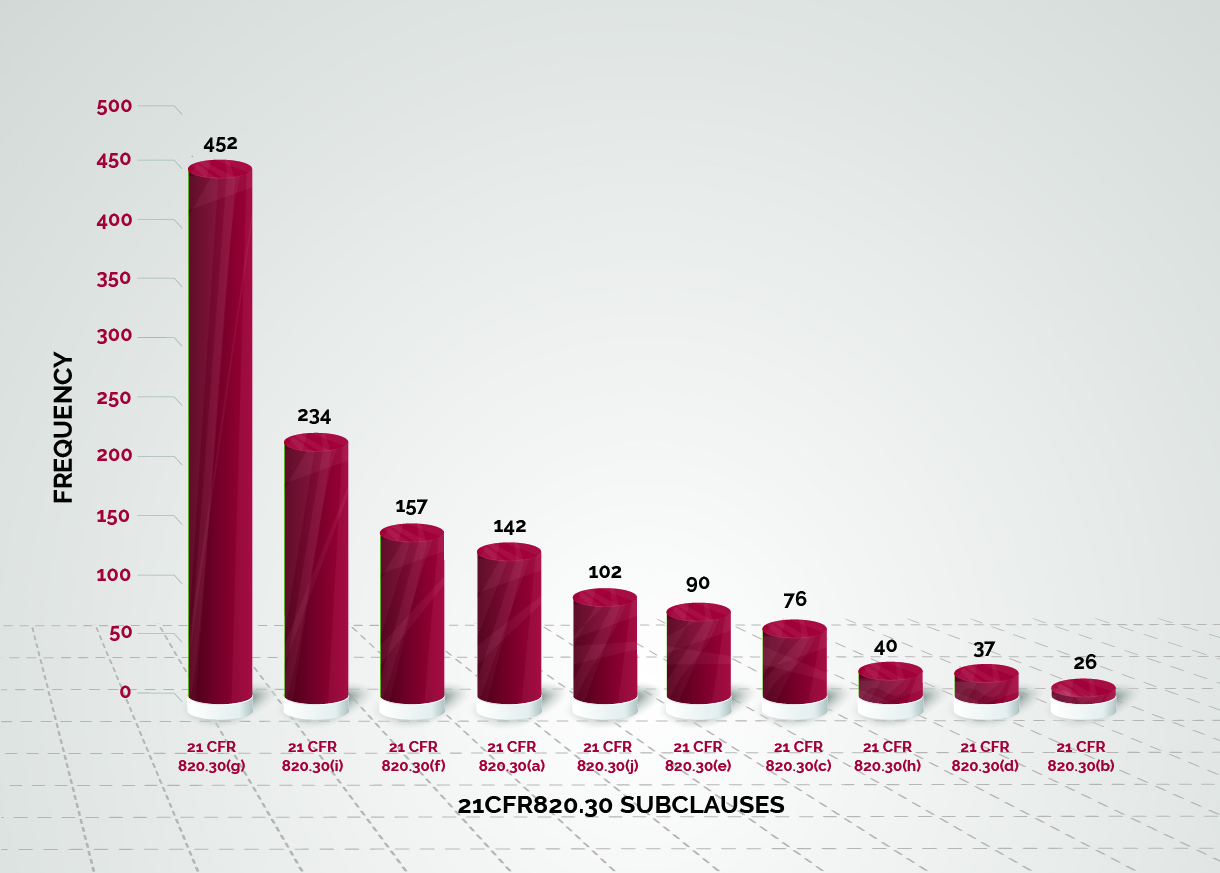



.webp?width=500&height=381&name=Quality%20management%20system%20product%20development%20(1).webp)